
Find noble gas in previous period - Cl is in period 3, Ne is the noble gas in period 2.So, if you add up all of the superscript numbers, you'll get 2 + 2 + 6 + 2 + 5 = 17 electrons.Ībbreviated ground state electron configuration Notice the number of electrons are written on top right of each sublevel.
Since we have 17 electrons and now that we know max number of electrons each sublevel can hold, we'll start from 1s and work our way up.
#Ground state electron configuration full
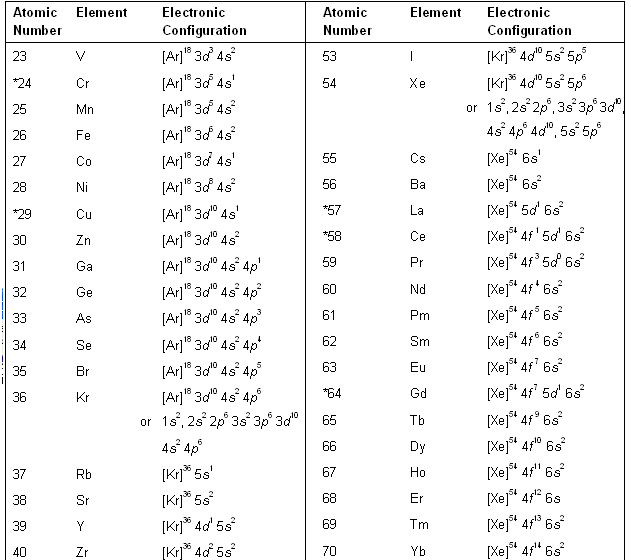
Įlectrons are filled up from the lowest energy level to the highest. full ground state electron configuration: 1s2 2s2 2p6 3s2 3p5 abbreviated: 'Ne' 3s2 3p5 Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form.
3d, has 5 orbitals, therefore, max of 5 x 2 = 10 electrons. Valency and Valence Electrons The outermost orbital shell of an atom is called its valence shell, and the electrons in the valence shell are valence electrons. The configuration is determined by applying the rules of the Aufbau Principle. 3p, has 3 orbitals, therefore, max 6 electrons. Figure : The ground state electron configuration of carbon, which has a total of six electrons. 3s, has 1 orbital, therefore, max 2 electrons. 2p has 3 orbitals, and therefore can hold max of 3 x 2 = 6 electronsįor energy level 3, there are 3 sublevels, 3s, 3p and 3d. 2s, only has 1 orbital, therefore 2s at most will hold max of 2 electrons. įor energy level 2, there are 2 sublevels, 2s and 2p. Since 1 orbital can hold at most 2 electrons, therefore 1s can hold max 2 electrons. We'll need to know how many sublevel is present in each energy level, and in turn, how many electrons each sublevel can accommodate.įrom the given table, for energy level 1, there's only 1 sublevel, which is called 1s. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form.






 0 kommentar(er)
0 kommentar(er)
